Could Sodium-Ion Batteries be the new king of energy storage?

For a long time, the world has relied on Lithium-Ion batteries for energy storage, but research into Sodium-Ion batteries has been gathering pace over the last decade and that should surprise no one.
Lithium-Ion batteries are unmatched in their performance, but in a world where some of the raw materials required to meet our energy production, storage, and transportation demands are rapidly becoming scarce and expensive, a new champion material must arise to meet the sustainable and clean energy storage challenges that have been presented to us. And this champion could be the Sodium-Ion battery.
Sodium is a soft, silvery-white, highly reactive alkali metal. It belongs in group 1 of the periodic table, and its atom has a single electron in its outer shell that it readily donates, creating a positively charged atom—the Na+ cation.

Alone, it’s explosive. Combined with chlorine, it’s table salt.
That’s sodium for you — a wild and wooly element that reacts easily and mixes with other elements to make some of the most common substances in daily life. Other than table salt (NaCl), sodium shows up in baking soda (NaHCO3), sodium peroxide (Na2O2) and borax, or sodium borate (Na2B4O7•10H2O). It’s crucial for blood-pressure control and the functioning of the nervous system.

In the mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. This relationship between the external load and the ability of the material to withstand this load is what gives us the Ultra Tensile Strength UTS, Yield strength, and its modulus of elasticity.
The hardness is the ability of the material involved to withstand surface indentation (localized plastic deformation) and scratching. This is where we get the Brinell Hardness (a spherical indentation test) and Mohs Scale ( the ability of one natural sample of mineral to scratch another mineral visibly.) results.

The Sodium-Ion Battery (SIB)
The sodium-ion battery (NIB or SIB) is a type of rechargeable battery analogous to the lithium-ion battery but uses sodium ions (Na+) as the charge carriers. Its working principle and cell construction are almost identical to those of commercially widespread lithium-ion battery types, but sodium compounds are used instead of lithium compounds.
Sodium-Ion Batteries were first developed in the 1980s alongside the Lithium-ion batteries, however, by the 1990s it was clear that Lithium-Ion batteries performed much better than their Sodium counterparts. Beyond performance, Sodium-Ion Batteries could not use some of the technologies and materials that their Lithium-Ion counterparts made use of. For example, due to its large size in comparison to the Lithium-ion, a graphite anode could not be used as the ions could not fit into the interstices within the carbon structure of the graphite anode.
Nonetheless, there has been renewed interest in SIBs as the world moves towards a more sustainable, environmentally, and cost-friendly alternative that can take over the energy storage needs of an increasingly growing power-hungry world as the Lithium-Ion batteries are slowly phased out.
The material makeup and operation of a sodium-ion battery
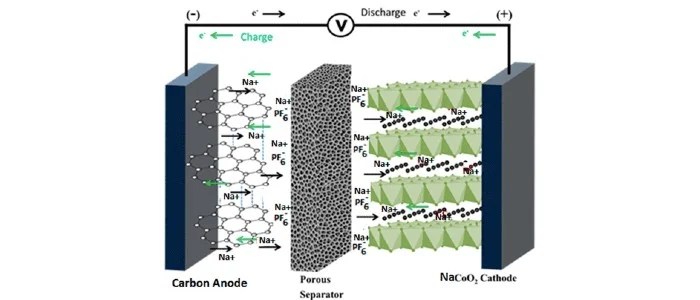
Sodium-ion battery cells consist of a cathode based on a sodium-containing material, an anode (not necessarily a sodium-based material), and a liquid electrolyte containing dissociated sodium salts in polar protic or aprotic solvents.
During charging, sodium ions are extracted from the cathode and inserted into the anode while the electrons travel through the external circuit; during discharging, the reverse process occurs where the sodium ions are extracted from the anode and re-inserted in the cathode with the electrons traveling through the external circuit doing useful work.
The Anode

Because the use of a graphite anode is not suitable for the large sodium ions, a disordered carbon material that is non-crystalline and of an amorphous carbon structure (called “hard carbon“)[1] is the current preferred sodium-ion anode of choice.
Hard carbon’s sodium storage was initially discovered in 2000. This anode was shown to have a storage performance that is similar to that seen for lithium storage in graphite anode for lithium-ion batteries
While hard carbon is clearly the most preferred anode due to its excellent combination of high capacity, lower working potential, and good cycling stability, there have been a few other notable developments in lower-performing anodes[2]
The Cathode
Significant progress has been achieved in devising high energy density sodium-ion cathodes since 2011. Different cathode materials for SIBs include layered oxides[3], polyanionic compounds[4], and Prussian blue analogs (PBAs)[5], among them, PBAs are literally the coolest and have attracted tremendous attention because their open framework structure could easily accommodate Na+ and enable its fast transportation.
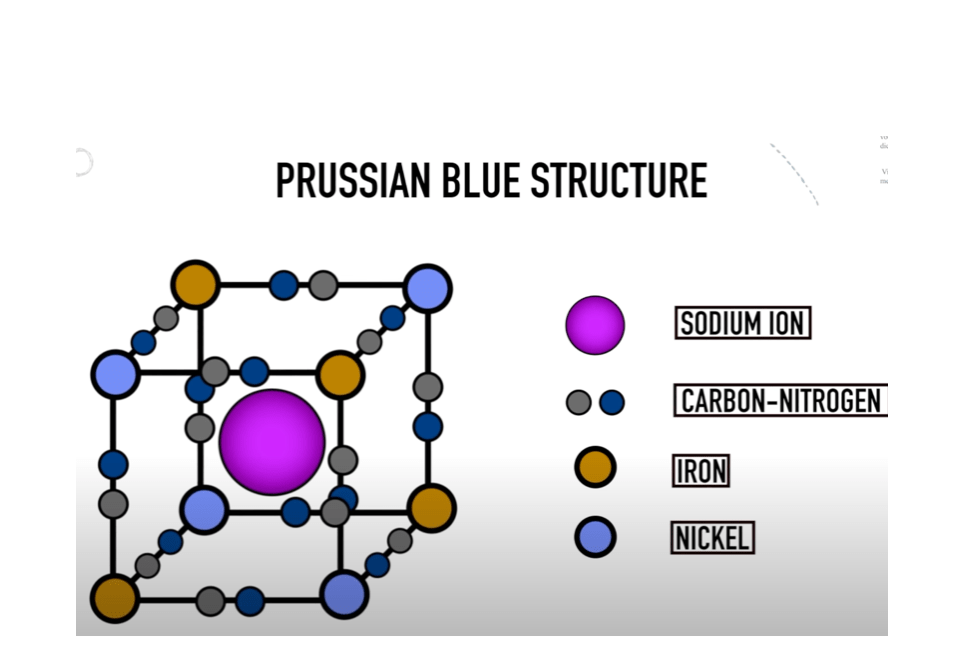
Compared with other cathodes, high-temperature calcination is not required during the synthesis of PBAs, which effectively lowers the manufacturing costs. These advantages make PBAs quite likely to be mass-produced and widely used as low-cost cathodes material for SIBs in the future.
The Electrolyte

Sodium-ion batteries can use aqueous as well as non-aqueous electrolytes. However, to improve the performance of the sodium-ion battery, a non-aqueous carbonate ester (polaric) may be used as a solvent. The current most widely used non-aqueous electrolyte uses sodium hexafluorophosphate[6]
My conclusion on the Sodium-Ion Battery and its importance to energy storage

First of all, table salt, like sodium, is a ubiquitous resource found naturally occurring in the earth’s crust and is more readily available compared to Lithium. This is an especially important point to note as Lithium is amongst the 1% of other metals that make up the earth’s crust. This means that it is not a readily abundant resource compared to sodium.
Secondly, Sodium-Ion batteries do not need cobalt, which is one of the increasingly scarce and expensive materials to mine from the earth. This means that sodium-ion batteries have the potential to be much cheaper per kg and per kWh in the long run compared to Lithium-Ion Batteries.
Moreover, Sodium-Ion batteries are much safer compared to Lithium-Ion battery, which uses more organic electrolytes that are highly flammable. Every year we hear a similar version of the same headline, recalls of particular tech products, fires, explosions, etc. Sodium-Ion batteries do not suffer from this challenge and are much more resilient; cut it, pierce it, or even shoot it and it stays intact.

Nonetheless, Sodium-Ion batteries have a long way to go, consider the chart below:

Compared to the extensively-used Lithium-ion batteries, Sodium-Ion batteries have a lower specific energy density and cycle life but perform better in a wide operational temperature range and are safer.
Sodium-Ion batteries have a similar working principle to Lithium-ion batteries and are expected to be at least 20% cheaper. In fact, by comparison, Sodium-Ion batteries cells are expected to be less sensitive to rising material costs from lithium, cobalt, and nickel. If all material prices rise 10%, Sodium-Ion batteries material costs will only increase 0.8%, while LFP and NMC 532 costs will increase 3.2% and 4.6%, respectively.
Sodium-Ion batteries will continue to be out of reach to the common man for the next couple of years as more time, resources and research continues to be poured into the development of this fairly new material. Despite some of its current setbacks, Sodium-Ion batteries remain the strongest candidate for use in energy storage, especially on a large national power grid-scale and commercially in consumer technology gadgets and electric vehicle manufacture.
Furthermore, Because some renewable energy technologies–such as wind and solar–have variable outputs, storage technologies are much needed to smooth out the electricity supply from these sources and ensure that the supply of power generated matches the demand. If charged during periods of excess renewable generation and discharged at times of increased demand ie at night, energy storage can help maximize the use of renewable energy and ensure that less is wasted.
Energy storage is also valued for its rapid response–battery storage can begin discharging power to the grid very quickly, within a fraction of a second, this is important for ensuring the stability of the grid when unexpected increases in demand occur.
Lastly, energy storage also becomes more important the farther you are from the electrical grid. Homes in rural communities that are farther away from the transmission grid are more vulnerable to power disruption than homes in large metropolitan areas. Because they may not be able to rely entirely on the larger grid, these communities can use energy storage to avoid blackouts thereby providing greater benefits to communities across the world while helping ease the impacts of pollution and climate change.
If the cost of Sodium-Ion batteries is further reduced, they will be favored for grid storage and home storage, where battery weight is not important. If, in addition to cost improvements, the energy density is increased, the batteries could be used for electric vehicles and power tools, and essentially any other application where lithium-ion batteries currently serve.
References and Resources
- Au H, Alptekin H, Jensen A, Olsson E, A O’Keefe C, Smith T, Crespo-Ribadeneyra M, Headen T, Grey C, Cai Q, Drewb A, Titirici M, 2020, A revised mechanistic model for sodium insertion in hard carbons, Energy & Environmental Science.
- Dahn, J. R.; Stevens, D. A. (2000-04-01). “High Capacity Anode Materials for Rechargeable Sodium‐Ion Batteries”. Journal of the Electrochemical Society. 147 (4): 1271–1273. Bibcode:2000JElS..147.1271S. doi:10.1149/1.1393348. ISSN 0013-4651.
- Yan, Z. et al. A hydrostable cathode material based on the layered P2@P3 composite that shows redox behavior for copper in high-rate and long-cycling sodium-ion batteries. Angew. Chem. Int. Ed. 58, 1412–1416 (2019). CAS Article Google Scholar
- Chen, M. et al. NASICON-type air-stable and all-climate cathode for sodium-ion batteries with low cost and high-power density. Nat. Commun. 10, 1480 (2019).Return to ref 6 in article ADS Article Google Scholar
- Qian, J. et al. Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv. Energy Mater. 8, 1702619 (2018).Return to ref 8 in article Article Google Scholar
- Sodium Hexafluorophosphate CAS #: 21324-39-0 Linear Formula: NaPF6 MDL Number: MFCD00011122 EC No.: 244-333-1
- Wang, W., Gang, Y., Hu, Z. et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat Commun 11, 980 (2020). https://doi.org/10.1038/s41467-020-14444-4
- Grid-Scale Battery Storage: Frequently Asked Questions
- Energy Storage | How It Works and Its Role in an Equitable Clean Energy Future
- Non-graphitizing Carbons P.J.F. Harris, in Encyclopedia of Materials: Science and Technology, 2001
- Sodium-protons-neutrons-electrons-electron-configuration
- Sodium – Periodic Table – Atomic Properties
- Sodium is the new lithium: Researchers find a way to boost sodium-ion battery performance
- Will sodium-ion battery cells be a game-changer for electric vehicle and energy storage markets?


