Gene Drive and Malaria!

‘the researchers had hacked the rules of inheritance’
Vox Docs
- Malaria Facts: Source: WHO
- Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes. It is preventable and curable.
- In 2017, there were an estimated 219 million cases of malaria in 87 countries.
- The estimated number of malaria deaths stood at 435 000 in 2017.
- The WHO African Region carries a disproportionately high share of the global malaria burden. In 2017, the region was home to 92% of malaria cases and 93% of malaria deaths.
- Total funding for malaria control and elimination reached an estimated US$ 3.1 billion in 2017. Contributions from governments of endemic countries amounted to US$ 900 million, representing 28% of total funding.
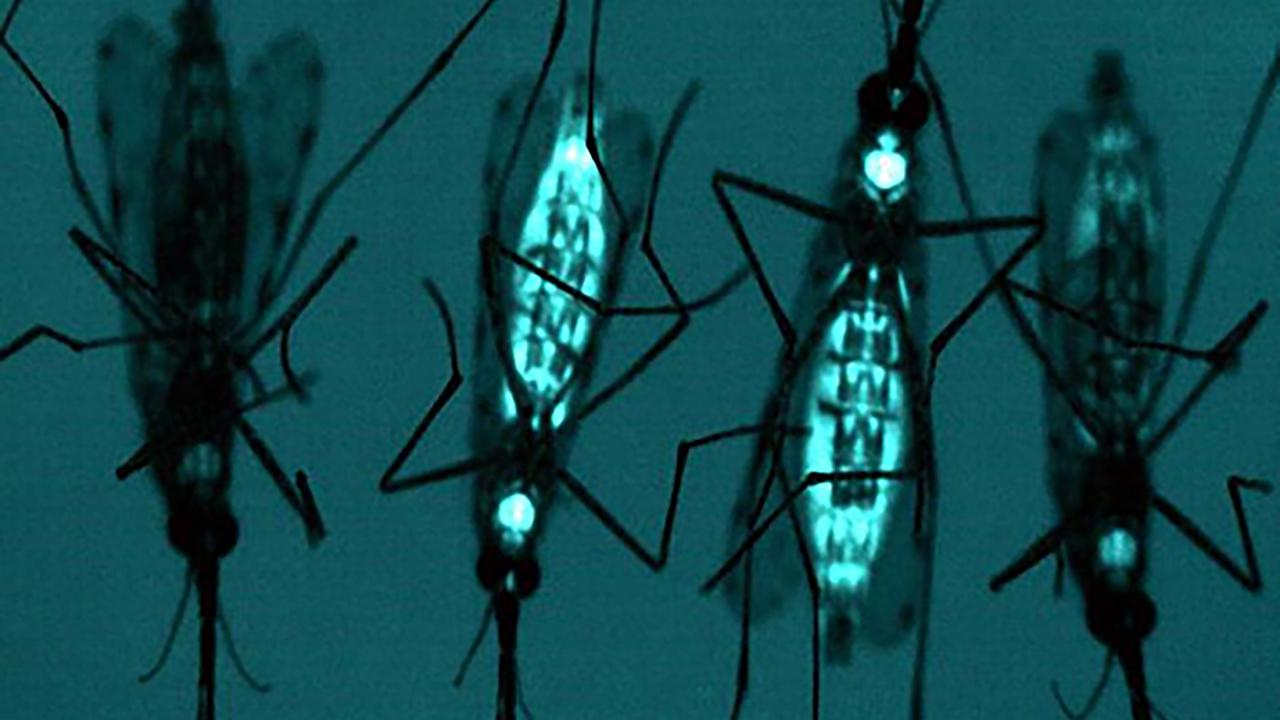
You see those mosquitoes up there those aren’t just any ordinary mosquitoes those mosquitoes are made to glow blue in variant laser light.
But that fluorescence is just a marker, it shows that something profound happened to those mosquitoes and their larvae. They have been genetically engineered to glow that way under that particular light.
Now this is not any normal success because those mosquitoes are the anopheles gambiae these mosquitoes are known to transmit malaria.
Now, only one of the parents of the mosquitoes only had the gene unit, you’d expect that only 50% of the mosquitoes would have the gene unit. But what is even more profound is that all the offspring 100% of that mosquito had the same gene copy across all of them. These scientists had indeed hacked the secret to gene drive biology.
CRISPR -Cas9

CRISPR is a gene editing tool that has been recently developed. It allows scientists to make precise edits to gene structures in micro-organisms such as viruses and bacteria, to the complex structures of humans and even mosquitoes. Gene drive on the other hand allows us to completely change the genetic composition of organisms by affecting only few individuals who then continue the transfer of this modification onto its subsequent generations.
This tool is very powerful and is hence what holds the future for eradicating malaria off the surface of the earth and therefore saving more children all over the world!
In this case the goal is to completely change the genetic composition of the malaria transmitting species of mosquitoes–Anopheles gambiae, Anopheles coluzzii, and Anopheles arabiensis– by use of the gene drive technology that hence allows for these species to be resistant to contracting the malaria infecting parasite.
CRISPR gene drives combine the gene drive idea with the CRISPR gene editing technique. They were first proposed in a 2014 paper — and the world of malaria research immediately realized the opportunity the idea presented. Target Malaria, a research consortium uniting Imperial College London with partner institutions in Burkina Faso, Mali, Uganda, and Ghana, is currently working with much more limited genetic engineering techniques to fight the disease. But according to engagement manager Delphine Thizy, they are hoping to apply for approval to test gene drives in the field as soon as 2023. They released an early non-gene drive mosquito in Burkina Faso in June 2019 as preparation
The science behind the tool, while not quite ready for release, is very, very, very close. I asked Ethan Bier, a professor at UC San Diego and one of the first people to help build an actual working CRISPR gene drive, how soon a drive targeting malaria could be released, as a scientific matter.
-Vox Observatory
Bier hesitated, and stressed that we shouldn’t release anything without regulatory approval and much more consideration. But he concluded, “To be honest with you, if there were some kind of emergency and one absolutely needed to do it, we could pretty much do it.”
And Esvelt, whose work helped pave the way for Target Malaria’s efforts, is terrified, simply terrified, of a backlash between now and then that could derail it. This is hardly a theoretical concern. In 2002, anti-GMO hysteria led the government of Zambia to reject 35,000 tons of food aid in the middle of a famine out of fear it could be genetically modified. Esvelt knows that the CRISPR gene drive is a tool of overwhelming power. If used well, it could save millions of lives, help rescue endangered species, even make life better for farm animals.
If a mistake was made to the gene drive system, their could be a potential mutation that would cause a worse effect than that present. Thus, a bad rapport for this potentially successful technology.
So how does CRISPR-Cas9 Gene Drive Work?
It works by inserting the gene editing tool itself into the target mosquito’s DNA from there CRISPR induces the cell to copy the package to the matching chromosome of the mosquito.
Like us most of us, mosquitoes have one pair of chromosomes and now that the gene drive has copied the package on to the two chromosomes then every subsequent offspring of that mosquito will have the same modification and it goes on and on and on!
So depending on what is attached to the gene editing tool like the blue fluorescent gene then every mosquito down the line will have the same nature as its parents.
This is very important for the drastic measure to combat the malaria causing parasite.
The team at the Imperial College London mainly catalyzing this research is part of a foundation called Target Malaria that are carrying out intense malaria projects to see both malaria come to an end. This organization is mainly funded by the Bill & Melinda Gates Foundation and they are aiming to stop malaria infection and suppress it using the gene drive technology.
Their drive is mainly focused towards ensuring female infertility that is less female anopheles mosquitoes in the entire mosquito population.
Then there is another team at the University of California whose main aim is to enhance the mosquitoes such that they cannot be able to contract the malaria causing parasite. Thus, not being able to transmit it between humans.
The trade off
The big tradeoff is that we need to carry out rigorous testing to ensure that it works.
Hence, we need to do it right because we need to save the kids. We need to be able to be certain and save the children now. That means ensuring this genetic technology is right and travels across borders.
For now, there are Target Malaria stations all over the world and Africa that are ensuring that the spread of malaria is contained using none genetically modified mosquitoes.
The Big Fear
The Big Fear is that the politics of genetically modified crops will spill into this great accomplishment about to be realized.
All in all the biggest barrier is nature itself but we have been able to take risks before and being able to eradicate Small Pox for example involved messing up with nature. At the end of the day we need to be able to overcome this barrier in order to successfully see malaria come to an end.
Read More
Michael Specter‘s article on Rewriting the Code of Life
The Extinction Invention Antonio Regalado‘s article
A simple guide to CRISPR, one of the biggest science stories of the decade by Brad Plumer, Eliza Barclay, Julia Belluz, and Umair Irfan
Brad Plumer‘s Gene Drive article on the tool that could transform the world!
Videos resources:
Vox Observatory’s The bold Plan to end malaria with a gene drive
Kurzgesagt-In a Nutshell’s Genetic Engineering & Diseases-Gene Drive & Malaria

Very eye opening,take it to the next level
LikeLiked by 1 person
In deed I am hoping to grow more and get more contributions from you guys, my dear readers
LikeLike
Nice and informative article. The issue with CRISPR technology and the potential backlash are of concern especially regarding the possible application to human beings and the terrifying risks involved
LikeLiked by 1 person
That is especially key, the fact that we need to be very certain when applying this radical gene drive technology. That is why loads of research and testing is being done to ensure the right stuff is done.
LikeLike
This is really nice
Good job👍
LikeLiked by 1 person
Thank you so so much for your support! Fell free to ask questions or even suggest topics you would like to see!
LikeLike